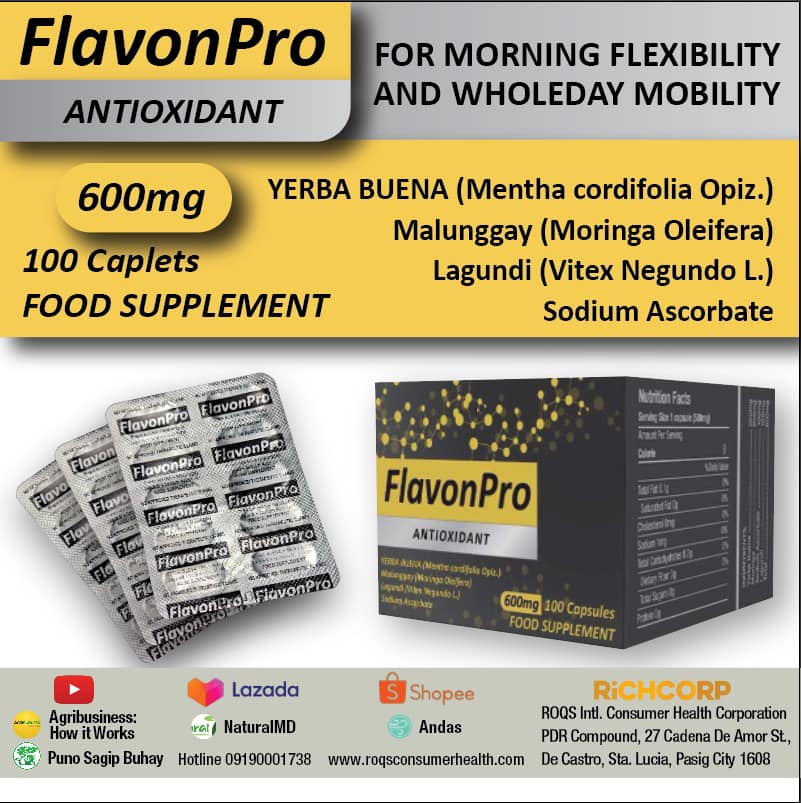
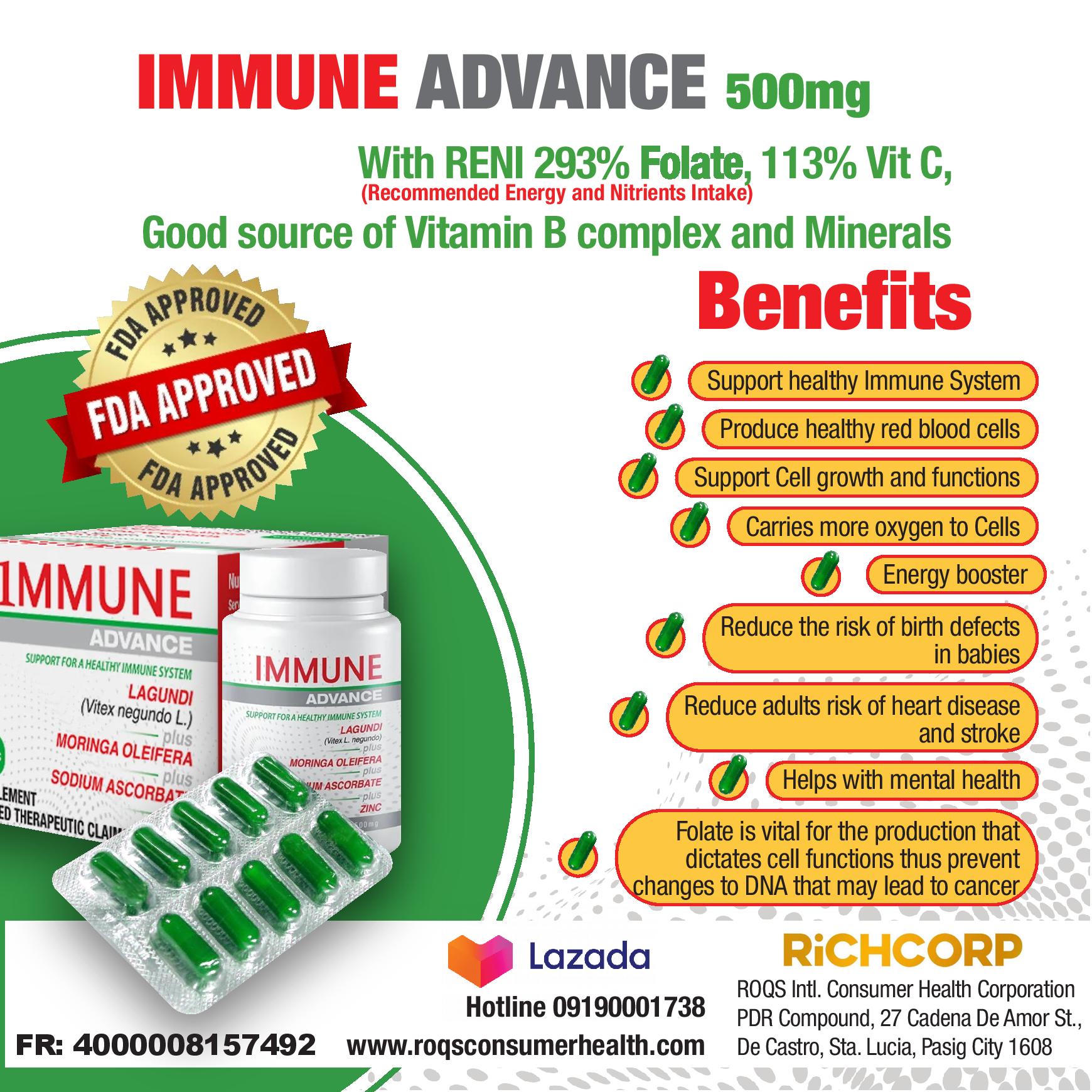
Boosting with AstraZeneca’s vaccine and mRNA COVID-19 vaccines provide equally high protection against Omicron-related severe outcomes, including hospitalization and death, even as new subvariants of the virus emerge, according to an expert review of more than 50 real-world studies.
The newly published review demonstrates that any three-dose schedule including the AstraZeneca vaccine was highly effective at protecting against severe Omicron outcomes (84.8%-89.2%*). Three dose schedules including mRNA vaccines showed equivalent effectiveness.1
The review authors conclude that the administration of a fourth dose booster is likely to add a significant level of additional protection, with a recent real-world study from Asia demonstrating no cases of severe outcomes due to Omicron in people vaccinated with a fourth dose of either the AstraZeneca vaccine or a mRNA COVID-19 vaccine during the February to April 2022 analysis period.2
Dr. Rontgene Solante, one of the review’s authors, said: “Current vaccines are still effective in protecting the vulnerable populations. Instead of focusing our resources on getting variant specific booster vaccines, it is important that all governments and all countries will focus on how to increase vaccination coverage and how to increase uptake of booster vaccination in the population.”
The 22 independent international infectious disease experts from across Asia and Latin America involved in the review concluded that a sustainable annual boosting strategy could include once a year boosting for the general population, and every six months for vulnerable groups, such as those living with chronic conditions.1

His personal research interests focus on severe bacterial infections, including meningitis and Staphylococcus aureus bloodstream infection, and tuberculosis. He has a longstanding research interest in the diagnosis, treatment and pathophysiology of tuberculous meningitis. Much of his research has been centred on large, pragmatic, randomized controlled trials which have addressed questions of key clinical importance, but have also provided the framework for providing unique insights into disease pathogenesis, antimicrobial pharmacology, and host and bacterial genetics. (Nuffield Department of Medicine, University of Oxford website)
Professor Guy Thwaites, Director of the Oxford Clinical Research Unit in Vietnam and one of the study’s authors said: “Booster dose data is critical for informing ongoing vaccination strategies as we transition from pandemic to endemic, whether that is an annual vaccine for most people, or every six months for those considered to be more vulnerable. This expert review of data can reassure governments and the public that viral vector and mRNA COVID- 19 vaccines offer great booster protection against serious outcomes in the ongoing battle against Omicron, particularly because that protection also shows very little sign of waning, even after a three-month period.”
The review, published online yesterday, analysed more than 50 global real-world studies hosted on ViewHub, a robust, interactive platform for visualizing global data on vaccine use and impact, developed by Johns Hopkins Bloomberg School of Public Health and the International Vaccine Access Center.
The reviewed data also showed that other vaccines used as boosters perform well against Omicron but appear slightly less effective than AstraZeneca’s vaccine and the mRNA COVID- 19 vaccines.1
AstraZeneca’s vaccine is a ‘viral vector’ vaccine, which means a version of a virus that cannot cause disease is used as part of the vaccine, so if the body is exposed to the real virus later it is able to fight it. This vaccine technology has been used by scientists over the past 40 years to fight other infectious diseases such as the flu, Zika, Ebola and HIV.3
AstraZeneca and its global partners have released over three billion vaccine doses to more than 180 countries, and approximately two-thirds of these doses have been delivered to low- and lower-middle-income countries. The vaccine is estimated to have helped save over six million lives during the first 12 months of use since December 2020, according to data from leading health analytics firm Airfinity.4# (Dominguez Marketing Communications, Inc.)
______________________
AstraZeneca COVID-19 Vaccine (ChAdOx1-S [Recombinant], formerly AZD1222) AstraZeneca’s COVID-19 vaccine was invented by the University of Oxford. It uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body.
The vaccine has been granted a conditional marketing authorisation or emergency use in more than 125 countries. It also has Emergency Use Listing from the World Health Organization, which accelerates the pathway to access in up to 144 countries through the COVAX Facility.
Under a sub-license agreement with AstraZeneca, the vaccine is manufactured and supplied by the Serum Institute of India under the name COVISHIELD.
In the Philippines, the COVID-19 Vaccine (ChAdOx1-S [recombinant]) COVAX Supply has been given ‘emergency use listing’ by the World Health Organization. Similarly, the direct supply has been granted Emergency Use Authorization (EUA) by the Philippine Food and Drug administration (FDA). This means that there is more evidence to come about this medicine. The World Health Organization and the Philippine FDA will review new information on this medicine as it becomes available, and the leaflet will be updated as necessary. Please also note the vaccine cannot be marketed or sold, advertised, and promoted.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
- Range in brackets shows that people who received three doses of COVID-19 vaccines (including AstraZeneca) are 84.8% to 89.2% less likely to fall seriously ill or die due to the virus, compared with unvaccinated populations. That means for every 100 unvaccinated incidents of severe illness or death, there are less than 16 cases among people who are fully vaccinated and boosted against COVID-19.
References:
1. Solante R, Alvarez-Moreno C, Burhan E et al. Expert Review of Global Real-World Data on COVID- 19 Vaccine Booster Effectiveness & Safety during the Omicron-dominant phase of the pandemic. 6 September 2022, REPRINT (Version 1) available at Research Square. https://www.researchsquare.com/article/rs-2015733/v1. Accessed September 2022.
2. Intawong K, et al. Heterologous third and fourth dose vaccine to reduce severity and mortality in COVID-19 patients during delta and omicron predominance: A cohort study in Chiang Mai, Thailand. Research Square 2022. Preprint published online, not peer reviewed, https://www.researchsquare.com/article/rs-1973470/v1. Accessed August 2022.
3. Sai V Vemula & Suresh K Mittal (2010). Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opinion on Biological Therapy, 10:10, 1469- 1487, DOI: 10.1517/14712598.2010.519332.
4. Data on file.